Rupture Test for Soft Gelatin Capsules USP Compliance
Soft gelatin capsules are widely used in the pharmaceutical industry for delivering medicines in a gelatinous form. To ensure their quality and efficacy, it’s crucial to perform various tests, one of which is the rupture test for soft gelatin capsules USP. This test is essential for evaluating the mechanical strength of soft gelatin capsules and ensuring they meet the required standards for durability and performance.
What Is the Rupture Test for Soft Gelatin Capsules?
The rupture test for soft gelatin capsules USP is a method used to determine the mechanical integrity of soft gelatin capsules under specific conditions. It simulates the mechanical stress that the capsule might experience during handling, storage, or transportation. The test involves applying pressure to a capsule until it ruptures, which provides valuable data on the capsule’s strength and potential vulnerabilities.
Why Is the Rupture Test Important?
This test is crucial for several reasons:
- Ensuring Quality: The rupture test helps assess the strength of the capsules, ensuring they do not break prematurely or fail during usage.
- Compliance with USP Standards: The test is part of USP guidelines that ensure the safety, reliability, and consistency of softgel capsules in pharmaceutical products.
- Formulation Adjustments: By analyzing rupture points, manufacturers can adjust the formulation and manufacturing process to improve capsule performance.
Key Methods for Conducting the Rupture Test
The rupture test for soft gelatin capsules USP typically involves the following steps:
- Sample Preparation: A number of soft gelatin capsules are selected for testing. These capsules should be free from any visible defects such as cracks or dents.
- Test Setup: The capsules are placed in a device capable of applying controlled pressure. This can be a softgel hardness tester, which provides precise pressure application.
- Pressure Application: Gradual pressure is applied to the capsule until it ruptures. The amount of pressure required to rupture the capsule is recorded.
- Data Analysis: The pressure data is analyzed to determine if the capsule meets the required USP standards for rupture force.
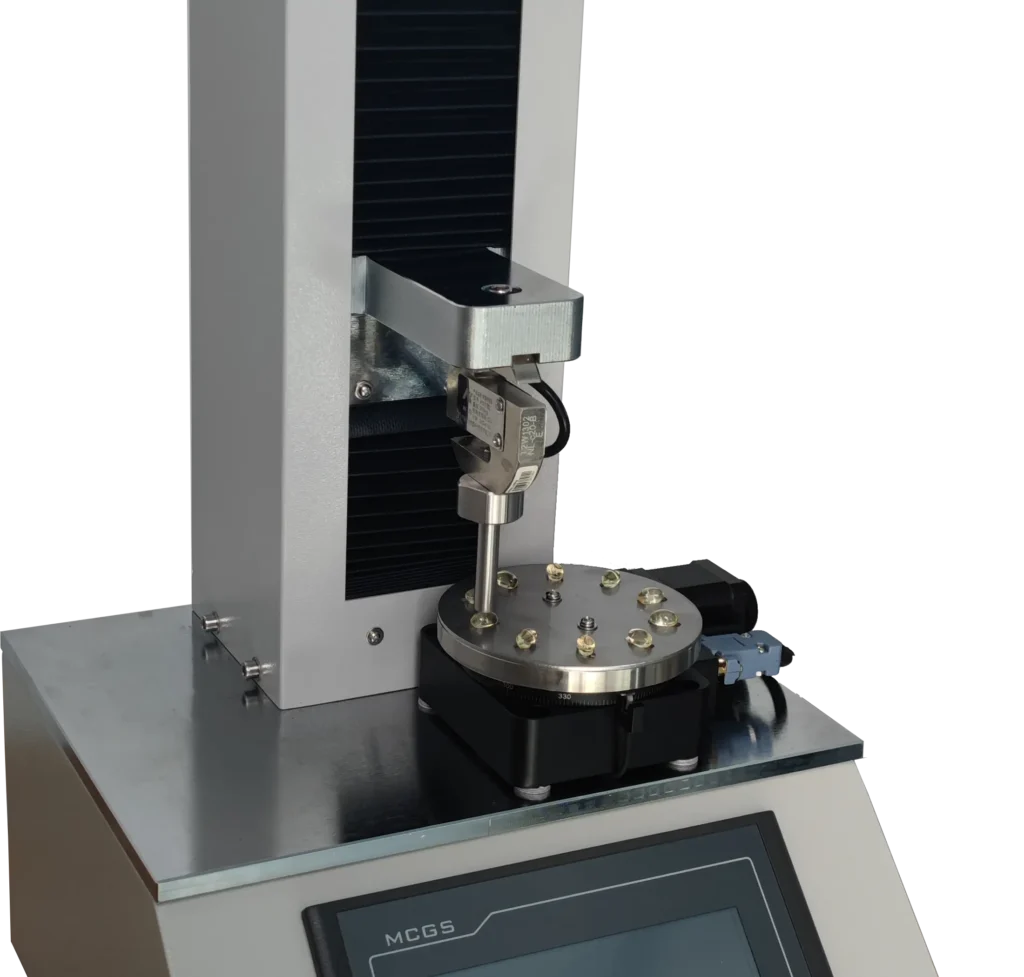
How Lab Softgel Hardness Testers Help in Rupture Testing
A lab softgel hardness tester is an essential instrument for conducting rupture tests on soft gelatin capsules. These devices are designed to apply uniform pressure to the capsules and measure their resistance to rupture. Key features of these testers include:
- Accurate Pressure Control: Lab testers can apply pressure at a consistent rate, ensuring reproducible results.
- Data Logging: Advanced softgel hardness testers can log data, allowing for detailed analysis of the capsule’s behavior under stress.
- Adjustable Testing Parameters: Operators can set specific test conditions such as force, speed, and duration to match the requirements of the USP rupture test.
By using a softgel hardness tester, manufacturers can ensure their products meet the stringent mechanical requirements set by regulatory bodies, ensuring better quality and safety for end-users.
Softgel Texture Analysis: Understanding Capsule Integrity
Softgel texture analysis is another critical aspect of ensuring the quality of soft gelatin capsules. While the rupture test measures the force required to break the capsule, texture analysis evaluates other aspects of the softgel, such as flexibility, elasticity, and overall integrity. These factors contribute to the overall stability of the capsule during its shelf life.
Softgel texture analysis involves assessing the physical characteristics of the gelatin shell, including its ability to stretch, compress, or bend under various conditions. This analysis is crucial for determining how the softgel will behave during manufacturing, storage, and eventual consumption. Specialized texture analyzers, often integrated with softgel hardness testers, can provide a comprehensive picture of the capsule’s quality.
By incorporating rupture testing for soft gelatin capsules USP into their quality control processes, pharmaceutical manufacturers can ensure the reliability and effectiveness of their products. Using specialized instruments like the lab softgel hardness tester enhances accuracy and helps comply with industry standards for optimal capsule performance.
